Prions, the misfolded proteins that are known for causing degenerative illnesses in animals and humans, may have been spotted for the first time in plants.
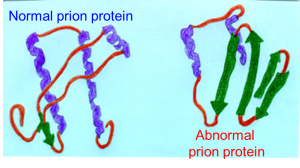 Researchers led by Susan Lindquist, a biologist at the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, report that they have found a section of protein in thale cress (Arabidopsis) that behaves like a prion when it is inserted into yeast.
Researchers led by Susan Lindquist, a biologist at the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, report that they have found a section of protein in thale cress (Arabidopsis) that behaves like a prion when it is inserted into yeast.
In plants, the protein is called Luminidependens (LD), and it is normally involved in responding to daylight and controlling flowering time. When a part of the LD gene is inserted into yeast, it produces a protein that does not fold up normally, and which spreads this misfolded state to proteins around it in a domino effect that causes aggregates or clumps. Later generations of yeast cells inherit the effect: their versions of the protein also misfold.
This does not mean that plants definitely have prion-like proteins, adds Lindquist — but she thinks that it is likely. “I’d be surprised if they weren’t there,” she says. To prove it, researchers would need to grind up a plant and see whether they could find a protein such as LD in several different folded states, as well as show that any potential prion caused a misfolding cascade when added to a test-tube of protein. Lindquist adds that because she’s not a plant scientist — her focus is on using yeast to investigate prions — she hasn’t tried these experiments. The study is reported on 25 April in the Proceedings of the National Academy of Sciences.
