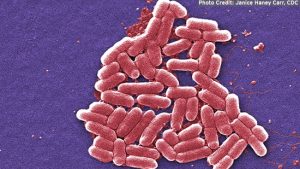
Enteroinvasive Escherichia coli (EIEC) and Shigella spp. are both Gram-negative bacteria causing diarrheal disease worldwide [1,2]. The clinical presentations of these two pathogens are very similar [3,4] and commonly manifested through diarrhoea, abdominal cramps, nausea and fever both in children and adults [5,6]. In addition to a similar clinical picture, EIEC and Shigella share laboratory features that can make it difficult to distinguish between them in routine clinical laboratory practice. Both pathogens are transmitted via the faecal-oral route and infections are frequently associated with consumption of contaminated food and water [7–10]. While Shigella is associated with large-scale food-borne outbreaks [11,12], outbreaks caused by EIEC are rarely recorded.
High prevalence of EIEC infections have been documented in rural areas and settings with poor sanitation in high-risk countries [5,13] while EIEC infections in Europe are typically sporadic and travel related [14]. Nevertheless, a few EIEC outbreaks have been reported in Europe, with the most recent ones having occurred in Italy in 2012 [15] and in the United Kingdom (UK) in 2014 [16]. These outbreaks affected 109 cases and 157 probable cases, respectively, highlighting the fact that EIEC, like Shigella, has the capacity to cause large gastrointestinal disease outbreaks. The outbreak strain identified in these recent European outbreaks, EIEC O96:H19, is an emergent type of EIEC that has phenotypic characteristics more resembling those of non-invasive Escherichia coli (E. coli) than those described for Shigella [17]. These characteristics are suggested to contribute to improved survival abilities as well as the ability to better adapt to different ecological niches [17].
Traditionally, culturing of faecal specimens has been the mainstay of laboratory diagnostics for enteric bacteria, and EIEC has been differentiated from Shigella by assessing a combination of several phenotypic characteristics, including biochemical, motility and serological traits [18,19]. This is now changing as PCR-based methods are becoming routine in many diagnostic laboratories [20]. In contrast to non-invasive E. coli, EIEC and Shigella can invade and multiply in intestinal epithelial cells [21], a process that is partially mediated by the products of the invasion plasmid antigen (ipa) genes [22]. For this reason, PCR targeting the ipaH gene can separate EIEC from other non-invasive E. coli, but cannot differentiate between EIEC and Shigella [23]. The lacY gene has been proposed as an additional molecular marker for which most E. coli are positive and Shigella is negative [24]. Its use as a PCR target in separating Shigella and EIEC is restricted to bacterial isolates since many faecal samples are lacY positive because of the presence of E. coli in the normal flora.
In Sweden, several clinical laboratories have shifted towards the use of direct PCR testing on faecal specimens as the primary diagnostic tool. However, most of these laboratories culture PCR-positive samples, so called PCR-guided culturing. Although culturing of PCR-positive faecal specimens is routinely performed, it can be difficult to obtain EIEC isolates since the morphology of EIEC strains on commonly used substrates can mimic the morphology of the enteric background flora, yellow colonies on xylose lysine deoxycholate (XLD) agar, rather than the morphology of Shigella, red colonies on XLD agar. Hence, separating EIEC from other bacteria in the normal flora usually whhttps://www.technologynetworks.com/applied-sciences/news/sticker-could-improve-safety-of-our-cold-chain-food-333138?utm_campaign=NEWSLETTER_TN_Food%20%26%20Beverage%20Analysis&utm_source=hs_email&utm_medium=email&utm_content=86254121&_hsenc=p2ANqtz–MMoS1KFFGpgRZ_seNnO0bmNz_SMVvraj4jqMu9SDGvKY_0jhrfEnmyzyUGUG4KMiZPINfu3qM8tMQOAJhBtcRodwdNw&_hsmi=86254121
Which is considered too time consuming for most clinical laboratories. For this reason, it is likely that a patient with specimens that are ipaH PCR-positive but culture negative would not be notified as a case if the diagnostic algorithm at the laboratory requires a detected Shigella isolate. In addition, PCR is a more sensitive method than culturing [25] and Shigella is known for its limited survival ability in faecal samples [26], which also may lead to samples being ipaH PCR-positive but culture negative.
Shigellosis is notifiable by law in Sweden as in the majority of countries in Europe [27]. In 2017, the incidence was 2.1 per 100,000 inhabitants in Sweden, and the majority of cases had been infected abroad [28]. The mandatory reporting of diseases allows the implementation of a series of public health actions, including public health management and surveillance activities, and helps define risk exposures. In contrast to shigellosis, reporting is not mandatory for EIEC and the occurrence of this pathogen in Sweden is currently unknown.It requires additional laboratory procedures such as screening large numbers of colonies,
Outbreak of gastroenteritis highlighting the diagnostic and epidemiological challenges of enteroinvasive Escherichia coli, county of Halland, Sweden, November 2017, 12 December 2019
Eurosurveillance
Nina Lagerqvist1,2, Emma Löf1,3, Theresa Enkirch1,2, Peter Nilsson4, Adam Roth1, Cecilia Jernberg1
https://doi.org/10.2807/1560-7917.ES.2020.25.9.1900466
https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.9.1900466