The U.S. Food Safety and Inspection Service (FSIS) is announcing the availability of its updated “Best Practices Guidance for Controlling Listeria monocytogenes (Lm) in Retail Delicatessens” and responding to comments received on the guidance that FSIS posted on its Web site and announced in April 2014 in the Federal Register.
 The best-practices guidance discusses steps that retailers can take to prevent certain ready-to-eat (RTE) foods that are prepared or sliced in retail delicatessens (delis) and consumed in the home, such as deli meats and deli salads, from becoming contaminated with Lm and thus a source of listeriosis. FSIS encourages retailers to review the guidance and evaluate the effectiveness of their retail practices and intervention strategies in reducing the risk of listeriosis to consumers from RTE meat and poultry deli products.
The best-practices guidance discusses steps that retailers can take to prevent certain ready-to-eat (RTE) foods that are prepared or sliced in retail delicatessens (delis) and consumed in the home, such as deli meats and deli salads, from becoming contaminated with Lm and thus a source of listeriosis. FSIS encourages retailers to review the guidance and evaluate the effectiveness of their retail practices and intervention strategies in reducing the risk of listeriosis to consumers from RTE meat and poultry deli products.
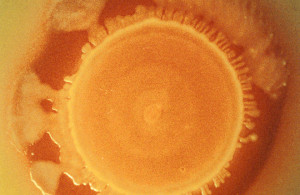
Lm is a bacterium that is found in moist environments, soil, and decaying vegetation and can persist along the food continuum. Transfer of the bacterium from the environment (e.g., deli cases, slicers, and utensils), employees, or contaminated food products is a particular hazard of concern in RTE foods, including meat and poultry products, because they generally receive no further processing for food safety before consumption. Listeriosis is a serious infection usually caused by eating food contaminated with Lm.
On April 21, 2014, FSIS announced the availability of its “Best Practices Guidance for Controlling Listeria monocytogenes (Lm) in Retail Delicatessens” and requested comment on the guidance (79 FR 22082). As explained in the 2014 Federal Register notice, FSIS used the key findings from the FSIS and Food and Drug Administration (FDA) “Interagency Risk Assessment–Listeria monocytogenes in Retail Delicatessens” available on FSIS’s Web site at http://www.fsis.usda.gov/wps/portal/fsis/topics/science/risk-assessments, the available scientific knowledge, the 2013 FDA Food Code, [1] and lessons learned from controlling Lm in FSIS-inspected meat and poultry processing establishments to develop the Best Practices Guidance for Controlling Lm in Retail Delis. The guidance provides practical recommendations that retailers can use to control Lm contamination and outgrowth in the deli. Retailers can use the best-practices guidance to help ensure that RTE meat and poultry products in the deli area are handled under sanitary conditions and are not adulterated under the Federal Meat Inspection Act (FMIA) (21 U.S.C. 601 et seq.) or the Poultry Products Inspection Act (PPIA) (21 U.S.C. 451 et seq.) (see 21 U.S.C. 623(d) and 464(e)). While these practices are specifically designed to control Lm, they also may help control other foodborne pathogens that may be introduced into the retail deli environment and other facilities where consumers take possession of food.
Final Guidance
The final guidance is posted at: http://www.fsis.usda.gov/wps/portal/fsis/topics/regulatory-compliance/compliance-guides-index.
 FSIS updated the guidance to replace the previous version of the document which was issued and announced in the Federal Register (79 FR 22082, April 21, 2014). FSIS updated this guidance based on comments received during the public comment period which closed on June 20, 2014. FSIS made the following changes to the guidance in response to comments: Clarified that food processing equipment should be disassembled during cleaning and sanitizing, added a recommendation that retailers scrub surfaces during cleaning to prevent biofilm formation, and clarified that retailers should rotate (change) sanitizers to help prevent Lm from establishing niches in the environment and forming biofilms. The response to comments section below contains a more detailed summary of the comments and FSIS’s responses to those comments. Although comments will no longer be accepted through www.regulations.gov on this guidance document, FSIS will update this document as necessary should new information become available.
FSIS updated the guidance to replace the previous version of the document which was issued and announced in the Federal Register (79 FR 22082, April 21, 2014). FSIS updated this guidance based on comments received during the public comment period which closed on June 20, 2014. FSIS made the following changes to the guidance in response to comments: Clarified that food processing equipment should be disassembled during cleaning and sanitizing, added a recommendation that retailers scrub surfaces during cleaning to prevent biofilm formation, and clarified that retailers should rotate (change) sanitizers to help prevent Lm from establishing niches in the environment and forming biofilms. The response to comments section below contains a more detailed summary of the comments and FSIS’s responses to those comments. Although comments will no longer be accepted through www.regulations.gov on this guidance document, FSIS will update this document as necessary should new information become available.
Response to Comments
FSIS received six comments on the “FSIS Best Practices Guidance for Controlling Lm in Retail Delicatessens” (FSIS Retail Lm Guideline). The comments were from a meat-processing company, a trade organization that represents retail stores, two companies that provide sanitation services, one company that produces antimicrobial agents, and one trade organization that represents meat-processing companies. The following is a summary of the comments that were received and FSIS’s responses to the comments.
Comment: Several commenters supported FSIS issuing the Retail Lm Guideline and recommended that FSIS issue other guidelines that retailers and food service operators can use. One commenter stated that the hazard of Lm does not change with production at a smaller facility and recommended that delis use the FSIS Compliance Guideline: “Controlling Lm in Post-lethality Exposed Ready-to-Eat Meat and Poultry Products” (FSIS Listeria Guideline). The FSIS Listeria Guideline is posted at http://www.fsis.usda.gov/wps/wcm/connect/d3373299-50e6-47d6-a577-e74a1e549fde/Controlling-Lm-RTE-Guideline.pdf?MOD=AJPERES.
Response: FSIS agrees that it is important to provide guidance for retailers and may issue additional guidelines as needed. While the FSIS Listeria Guideline for industry discussed in the preceding paragraph provides useful information about controlling Lm in federally inspected establishments, it does not provide information for deli operators. Because the requirements, processing conditions, and practices are different at retail than in processing facilities, issuing this separate guideline provides the specific information retailers can use to control Lm in the deli area.
Comment: Three commenters questioned whether the recommendation to rotate sanitizers to help prevent Lm from developing resistance to sanitizers and forming biofilms was necessary. One commenter stated that there is no scientific evidence that Lm develops resistance to sanitizers. The commenters recommended that retailers focus on removing the biofilm during the washing step and not the sanitizing step.
 Response: Research has shown that Lm may become resistant to chlorine and other sanitizers, [2] and several industry guidelines recommend rotating sanitizers. 3 4 5 6 Therefore, in the guidance, FSIS continues to recommend this practice to help prevent Lm from establishing niches in the environment and forming biofilms. FSIS agrees with the commenters that biofilm formation is a concern in the deli environment and should be addressed during the cleaning step. To address this concern, FSIS has added a new recommendation to scrub surfaces during cleaning to prevent biofilm formation.
Response: Research has shown that Lm may become resistant to chlorine and other sanitizers, [2] and several industry guidelines recommend rotating sanitizers. 3 4 5 6 Therefore, in the guidance, FSIS continues to recommend this practice to help prevent Lm from establishing niches in the environment and forming biofilms. FSIS agrees with the commenters that biofilm formation is a concern in the deli environment and should be addressed during the cleaning step. To address this concern, FSIS has added a new recommendation to scrub surfaces during cleaning to prevent biofilm formation.
Comment: One commenter recommended that FSIS compliance investigators treat the best practices as guidance and not regulatory requirements when performing in-commerce surveillance at retail. The commenter requested that FSIS instruct its compliance investigators that the best practices are recommendations and not requirements. The commenter also recommended that compliance investigators provide the retail store management with FSIS guidance and other guidance documents that are available if they determine that store management is not aware of Listeria control actions.
Response: FSIS agrees that the guidance represents FSIS’s best practices recommendations and does not represent requirements that retailers must meet. FSIS issued instructions to its compliance investigators to make them aware that this guidance did not include requirements. FSIS is not aware of any instance in which compliance investigators have enforced FSIS guidance as though it were a regulatory requirement. FSIS is instructing its compliance investigators through training materials that they should inform retailers that the guidance is available on the FSIS Web site. Retailers are required by the FMIA and PPIA to maintain sanitary conditions and otherwise not produce adulterated or misbranded product. The guidance provides actions retailers can take to help ensure that they are meeting the requirements of the FMIA and PPIA. Retailers also should be aware that the recommendations in the guideline, especially those based on the 2013 FDA Food Code, may be requirements in State, local, or Tribal regulations.
Comment: One commenter stated that it is important to disassemble equipment when cleaning to find hard-to-reach areas where Lm can hide. The commenter stated that FSIS should amend the recommendation to clean and sanitize RTE food-processing equipment every four hours to include recommendations to disassemble the equipment during cleaning.
Response: FSIS agrees that it is important to disassemble equipment (e.g., slicers) when cleaning every four hours as recommended by the 2013 FDA Food Code and has clarified this information in the guidance.
 A federal inspector on a routine visit to food service facilities at Los Angeles International Airport in January found conditions that, she wrote, could compromise the safety of food meant for airline passengers.
A federal inspector on a routine visit to food service facilities at Los Angeles International Airport in January found conditions that, she wrote, could compromise the safety of food meant for airline passengers.