Cryptosporidium parvum is a gastrointestinal parasite that can cause moderate to severe diarrhea in children and adults, and deadly opportunistic infection in AIDS patients.
 Because C. parvum is resistant to chlorine disinfectant treatment, it frequently causes water-borne outbreaks around the world. A study published on Nov. 12th in PLOS Pathogens provides a detailed analysis of a C. parvum protein that is central to glycolysis — the only pathway by which the parasite can generate energy — and identifies it as a potential drug target.
Because C. parvum is resistant to chlorine disinfectant treatment, it frequently causes water-borne outbreaks around the world. A study published on Nov. 12th in PLOS Pathogens provides a detailed analysis of a C. parvum protein that is central to glycolysis — the only pathway by which the parasite can generate energy — and identifies it as a potential drug target.
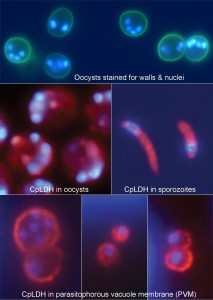
Guan Zhu and colleagues, from Texas A&M University in College Station, USA, study the parasite’s metabolism during its complicated life-cycle. C. parvum exists both in free stages (where parasites are in the environment or in the host’s digestive tract) and intracellular stages following host cell invasion, during which the parasite occupies a specialized compartment — the parasitophorous vacuole — which is delineated by a host-cell derived border called the parasitophorous vacuole membrane (PVM).
For this study, the researchers focused on lactate dehydrogenase (LDH), an enzyme central to glycolysis. Glycolysis is the only metabolic process by which organisms like C. parvum — that lack functional mitochondria to derive energy from oxygen — can generate ATP, the universal biological energy storage molecule. They found that the C. parvum LDH (CpLDH) protein is found inside the parasite’s cells during the free stages, but is then transferred to the PVM during intracellular development, indicating involvement of the PVM in parasite energy metabolism, and specifically, in lactate fermentation. They also demonstrate that two known LDH inhibitors, gossypol and FX11, can inhibit both CpLDH activity and parasite growth.
The researchers summarize that their observations “not only reveal a new function for the poorly understood PVM structure in hosting the intracellular development of C. parvum, but also suggest LDH as a potential target for developing therapeutics against this opportunistic pathogen, for which fully effective treatments are not yet available”. Acknowledging that the ultimate validation of CpLDH as a drug target requires tools for knockout or knockdown of genes of interest in Cryptosporidium, they say recent advances towards this goal raise hope that such validation will be possible in the near future.
Overall, they conclude that “the present data, together with the fact that C. parvum relies on glycolysis for producing ATP, support the notion that CpLDH is worth exploring as a potential target for the development of anti-cryptosporidial therapeutics.”
