The American Meat Institute has submitted comments recommending that USDA’s Food Safety and Inspection Service (FSIS) withdraw its proposed rule requiring labeling on needle- or blade-tenderized beef products.
“The existing labeling scheme for products that have been needle injected or blade tenderized, with appropriate qualifying statements or 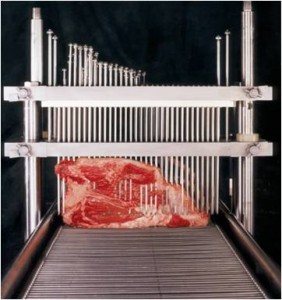 other label information, provides open and transparent information based on recognizable common and usual product names and should be kept,” the comments say.
other label information, provides open and transparent information based on recognizable common and usual product names and should be kept,” the comments say.
The comments highlight, among other things, the safety record of mechanically tenderized (MT) products, as well the proposed rule’s potential to confuse consumers by changing the product name to include the mechanically tenderized distinction.
AMI’s full comments are available at http://www.meatami.com/ht/a/GetDocumentAction/i/94617
.
